Research
3D Printing and Bioprinting with Artifical Intelligence
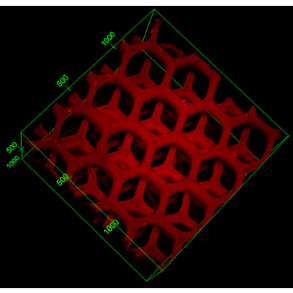
We are one of the leading groups in the field in developing photo-polymerization-based 3D printing and bioprinting techniques. Our printing methods are capable of creating 3D scaffolds in mere seconds with micro and/or nanoscale resolution in hydrogels. We pioneered the development of rapid 3D printing methods – “Dynamic Optical Projection Stereolithography (DOPsL)” (Advanced Materials, 2012) and “Microscale Continuous Optical Bioprinting (μCOB)” (Biomaterials, 2017). We initiated the concept of parallel and continuous polymerization during the 3D printing process and developed dynamic, optical, projection-style 3D printers. DOPsL and μCOB offer a printing speed that is thousand times faster than a traditional nozzle-based 3D printer.
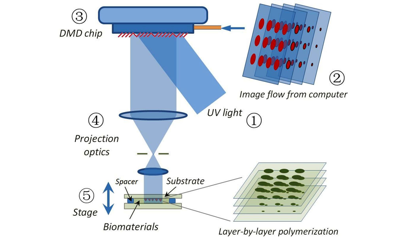
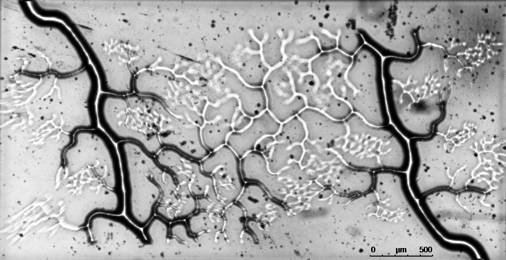
(Left) Schematic of the DOPsL method; (right) rapid printing of vascular network in 11 seconds.
Stem Cells and Tissue Engineering
In stem cell engineering, we are focusing on human induced pluripotent stem cells (iPSCs). iPSCs are a promising technology in regenerative medicine as they can be autologously derived, maintain high p roliferative capacity, and demonstrate enormous differentiation potential, while also mitigating the ethical concerns associated with the use of embryonic stem cells (ESCs). We are developing 3D scaffolds to study stem cell behavior in 3D micro-environments. Through precise control of spatial and temporal distributions of biological factors in 3D scaffolds, we are also investigating the interactions of stem cells with extracellular matrix (ECM) proteins, with the ultimate goal of creating advanced, clinically translatable tissue constructs. For example, using our rapid 3D printing method, we were able to create biomimetic scaffolds in mere seconds from MRI image to repair spinal cord injury (Nature Medicine, 2019, also highlighted in Nature Neuroscience, 2019, and NIH Director’s Blog). Currently, we are creating prevascularized tissues with complex 3D microarchitectures. Multiple cell types mimicking the native vascular cell composition are encapsulated directly into hydrogels with precisely controlled distribution. Creating vascularized tissues will lay the critical foundation for future large tissue and organ printing.
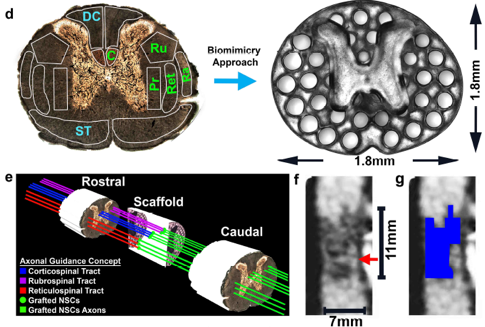
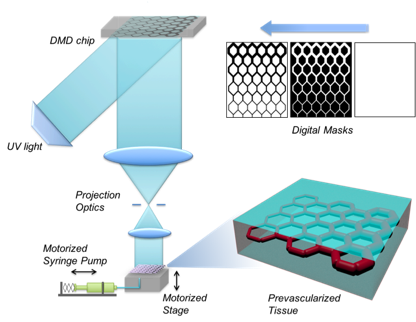
(Left) 3D-printed scaffold to repair spinal cord injury; (right) 3D-printing of prevascularized tissue.
Biomaterials and Nanomaterials
Over the years, we have been developing a library of biomaterials and bioinks including HA, GelMA, GelNB, PEGDA, PU, PGSA, PC, and decellularized ECM to meet the demands of bioprinting various tissue types such as liver, heart, nerve, muscle, cancer, retina, and placenta. Using 3D printing, we have created a variety of functional scaffolds. We showed that digitally patterned stiffness in hydrogel can control cell migration and differentiation. For the first time in the field, we reported that the Poisson’s ratio of hydrogel scaffolds can also be tuned and it can take on negative values (“auxetic”), an exception for most materials and structures. The auxetic behavior was achieved by patterning specially arranged and shaped unit-cellular structures using 3D printing. By encapsulating nanoparticles in the 3D-printed scaffolds, we have created a nano-detoxifier to remove toxins in blood (Nature Communications, 2014) and a self-propelled micro-robotic fish (Advanced Materials, 2015).
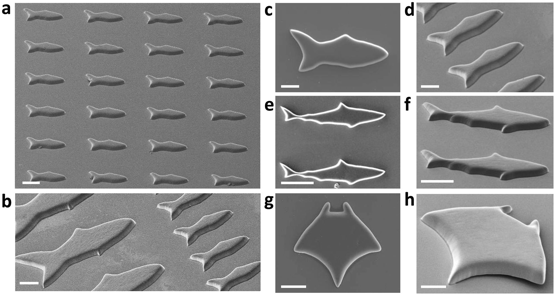
(Left) A nano-detoxifier to remove toxins in blood; (right) 3D-printed micro-robotic fish array.
Organ/Tissue-on-a-Chip
By integrating micro-fluidics with 3D printing, we are moving towards the creation of tissue-on-a-chip and organ-on-a-chip for disease modeling and early drug screening. As an example, we are the first group in the field in developing a deterministically patterned human iPSC-derived hepatic model using the DOPsL 3D bioprinting method (PNAS 2016, and Highlighted in Nature Reviews Gastroenterology & Hepatology, 2016). This biomimetic iPSC liver model has precise co-location of hepatic cells and supporting cells in a microscale 3D hexagonal architecture. We have also created an instrumented heart tissue that allows us to test cardio-toxicity and model cardiac diseases. Such precision human iPSC tissue models could revolutionize the fields of drug screening and disease modeling.
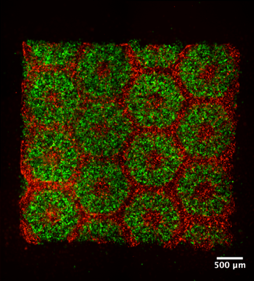
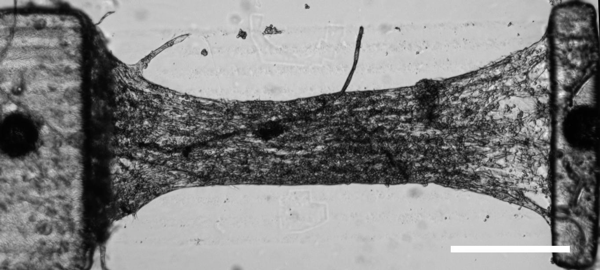
(Left) Bioprinted iPSC-derived human liver tissue; (right) instrumented heart tissue for drug screening.



